0 C being defined by the water freezing point and 100 C being defined by the water boiling point at standard atmospheric pressure. 610 m 208 ºF.
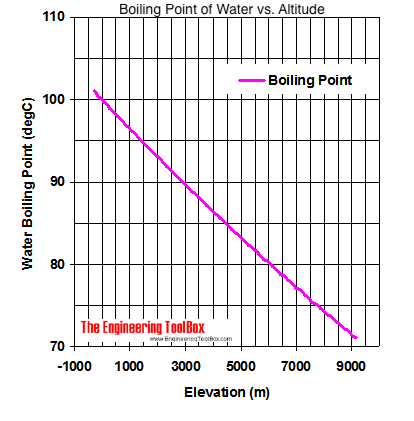 Boiling Point Of Water Vs Altitude
Boiling Point Of Water Vs Altitude
One atmosphere of pressure On Everest 8848 meters high the liquid boils at 86 degrees Celsius.
Water boils at what temperature celsius. Given the temperature in one scale we can. Its useful to know each amount. The temperature at which substance change from liquid to the gaseous state is known as the boiling point.
Water boils at 100 C or 212 F at one atmosphere of pressure. Though its one of the basic facts you probably learnt pretty early on back in school science lessons your elevation relative to sea level can affect the temperature at which water boils due to differences in air pressure. 0 m 212 ºF.
1000 ft 305 m 210 ºF. The Celsius temperature scale was defined until 1954 by two points. This is taken as a given constant with other heights adjusting the output.
1372 m 2035 ºF. The boiling point of a liquid varies depending on the external pressure. Take an example such as Mount Elbert Colorado the highest peak of the Rocky Mountains and the highest elevation point in the United States.
Phase diagram of water This shows the phase which is thermodynamically favourable at any given combination of temperature and pressure. Pure water boils at 100 degrees Celsius under normal atmospheric pressure but the Dead Sea surface is so low more than 400 meters below sea level that. We all learn at school that pure water always boils at 100C 212F under normal atmospheric pressure.
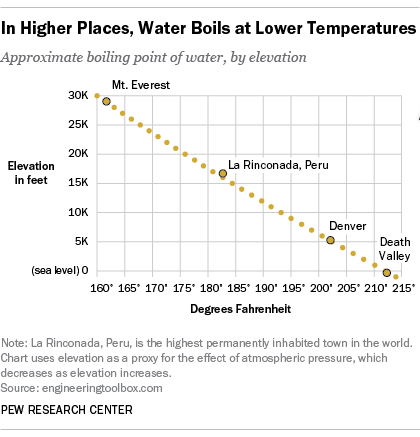
At sea level pure water boils at 212 F 100C. At the lower atmospheric pressure on the top of Mount Everest pure water boils at about 154 F 68C. 1219 m 204 ºF.
762 m 207 ºF. Water always boils at 100C right. Assuming standard barometric pressure the boiling point of water is.
The boiling point of water being 100 at standard atmospheric pressure since the freezing and boiling points will vary depending upon the pressure. Temperature given as C F K and R. 3000 ft 914 m 206 ºF.
152 m 211 ºF. When water is heated it reach a temperature - the boiling point - at which the vapor pressure is large enough that bubbles are formed inside the water. And removing dissolved air from water can easily raise its boiling temperature by about 10 degrees centigrade.
Boiling Point - Celsius. Average pressure at sea level pure water that is. When measuring temperature the usual units are Celsius degree Celsius or.
At sea level pure water boils at 212 F. 100 degrees Celsius or 212 degrees Fahrenheit. Answer100 degrees Celsius at standard pressureAt 1 atmosphere of pressure that is.
Pure water boils at 100 degrees Celsius under normal atmospheric pressure but the Dead Sea. Distilled water boils at 100º Celsius. At lower pressure or higher altitudes the boiling point is lower.
1067 m 2055 ºF. When the pressure of the atmosphere is reduced a liquid boils at a lower temperature. Online calculator figures and tables showing boiling points of water at pressures ranging from 147 to 3200 psia 1 to 220 bara.
Water at sea level always boils at the same temperature. What is the boiling point of water in Kelvin Celsius and Fahrenheit. What is The Boiling Point of Water.
We can answer this question by looking at the equilibrium phase diagram for water. Relation between the normal boiling point and the. Liquids boil when the pressure of the atmosphere is equal to the pressure of the liquid.
The boiling point of water varies with atmospheric pressure. You can boil water at. At an altitude of 19000 meters we cause the boiling point to change to 37ºC.
The boiling point of water in Celsius Fahrenheit and Kelvin scales are 100 degree Celsius or C 212 degree Fahrenheit or F and 373 Kelvin or K. If you look at the horizon. Water boils are 100 degrees Celcius 212 Fahrenheit.
457 m 209 ºF. Water boils at 3732 K Kelvin 100ºC Celsius or 212ºF Fahrenheit.