Pure sulfuric acid has a specific gravity of 1835 since it weighs 1835 times as much as pure water per unit. This is notable as wood shrinks about 815 as it dries.
How To Measure The Specific Gravity Of Rocks Roger Marjoribanks Roger Marjoribanks
Moreover in thi9s topic we will discuss specific gravity specific gravity formula and its derivation and solved examples.
What measures specific gravity. Molecular weights can be used to calculate Specific Gravity if the densities of the gas and the air are evaluated at. The reference material could be anything but the most common reference is pure water. The normal specific gravity of urine is 10031030 Specific gravity is measured by means of a hydrometer.
The solid wood density may be determined using the green volume the ovendry volume or intermediate volumes. The device is designed to float freely at the liquid surface with a protruding stem giving a reading corresponding to the specific gravity of the liquid. How does a refractometer measure specific gravity.
For liquids the usual standard is water. The specific gravity then may be determined by dividing the weight of a substance in grams by its volume in cc. Make sure that the liquid in the container is deep enough to allow the.
Typically water is used as a reference substance but other elements can be used as well based on the properties of the obect you are meas. Specific gravity is a measure of density relative to the density of a reference substance. A refractometer is an optical device that like a hydrometer measures the specific gravity of your beer or wort.
The gem purity can be determined by comparing its specific gravity with the already measured high purity level of. The specific gravity of pure water is 1000. Measuring Specific Gravity There is a wide range of instruments designed to measure the specific gravity of a material.
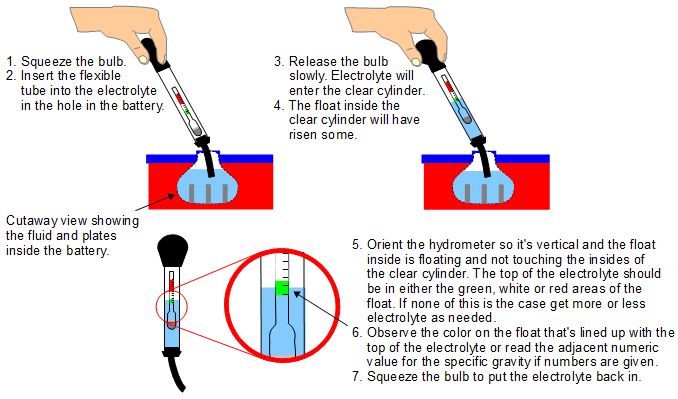
Lead-acid batteries use an electrolyte which contains sulfuric acid. NTP - Normal Temperature and Pressure - defined as 20 o C 29315 K 68 o F and 1 atm 101325 kNm2 101325 kPa 147 psia 0 psig 30 in Hg 760 torr. Specific gravity is a concept that we all have seen but do not know its name.
They then use these numbers to calculate usually using software what the ABV of their batch of beer is. It does so by sampling a small amount of liquid and looking at its optically. If a material has a specific gravity less than 1 it will float on water.
165 rijen In gemology specific gravity is usually determined through an apparatus. Specific gravity is the unit less ratio of the solid wood density to the density of water at the same temperature. What Is Specific Gravity.
Specific gravity is defined as the ratio comparing the weight of any liquid to the weight of an equal volume of water. Measuring Specific Gravity with a Hydrometer 1. Pour a sample of your liquid into a container.
The basic specific gravity always uses the green volume. Specific gravity is used by mineralogists and geologists to determine the mineral content. Most homebrewers measure the specific gravity of their beer at the beginning of fermentation and then at the end.
But it is more convenient in practice to determine it by dividing the weight of the substance by the weight of an equal volume of water. The hydrometer can be used to measure the specific gravity of any liquid. Specific gravity has a wide range of applications following are a few of the applications.
Also the density of the object determines this factor. If a sample of urine shows a specific gravity of 1025 this means that the urine is 1025 times heavier than water. The specific gravity of water is 1.
Specific gravity is ratio of the density of an object compared to the density of a known reference substance.
Specific heat formula Q m C Δ T. 360 Thermal Prope ADD.
 Heat Capacity Of Tungsten At Constant Pressure And Constant Volume The Download Scientific Diagram
Heat Capacity Of Tungsten At Constant Pressure And Constant Volume The Download Scientific Diagram
It is a measure of a substances ability to transfer heat through a material by conduction.
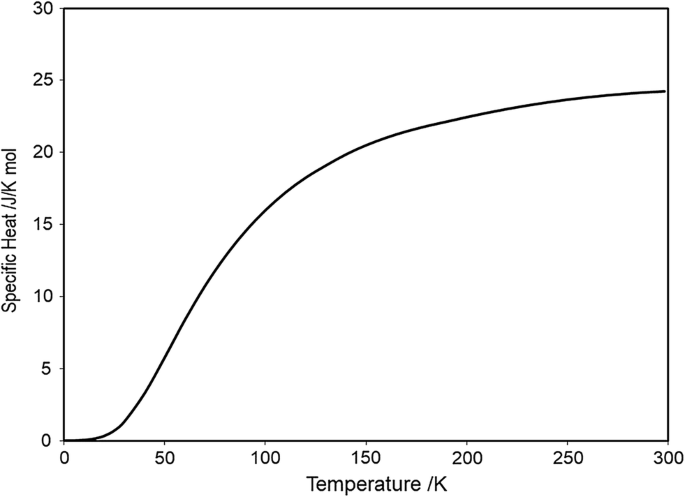
Specific heat of tungsten. The platinum crucible containing the. Heat Capacity JKg-K Temperature K Pressure Pa 200. Here Q is heat energy m is mass C is specific heat and Δ T is change in temperatureFrom the law of thermodynamics Q 1 Q 2 0.
In its most basic form tungsten carbide is a fine gray powder but it can be pressed and formed for use in industrial machinery cutting tools abrasives armor-piercing shells and jewellery. Thermal Properties of Mercury. 17 Standard Molar Entropy.
Tungsten carbide is a very dense carbide containing equal parts of tungsten and carbon atoms. Weight - The specific gravity of tungsten carbide is from 1-12 to 2 times that of carbon steel. At 3 420 C tungsten has the highest melting point of all metals.
I A complete analytical description of the tungsten specific isobaric heat capacity can be constructed by combining the WhiteMinges fit in the temperature range 300 TK. In the case of tungsten no change cf the contents of the crucible could be stated even af ter a prolongated heating of it at 1600 C. Modulus of elasticity of tungsten plotted against the testing temperature compared to our other refractory metals.
Latent Heat of Fusion. 1 Btu lb-F 41868 J kg-K 1 Btu lb-F 41868 J g-C 1 Btu lb-F 18 Btu lb-C Specific Heat Capacity of Metals Table Chart. Hot Hardness - When temperature increase to 1400F tungsten carbide retains much of its room temperature hardness.
Tungsten Thermal Conductivity. Initial temperature of tungsten T 2 i 100 C. The materials high thermal stability coupled with its high modulus of elasticity give tungsten its high creep resistance.
Thermal conductivity of Tungsten is 170 WmK. The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity k or λ measured in WmK. Where Q 1 and Q 2 is heat energy to different systems.
8 Zeilen Tungsten Specific Heat Latent Heat of Fusion Latent Heat of Vaporization. Specific Heat Capacity Conversions. The final heat-treatment temperature of materials except single-crystalline pure W was 900C for 20min.
Final temperature of tungsten and water T 1 f T 2 f 216 C. Specified Heat - Tungsten carbide ranges from about 50 to 70 as high as carbon steel.
CONVERSION CALCULATORS Convert between Different Units of Measurement. 23 456 123 45 Material Density.
 Specific Gravity Definition Calculation Solved Examples Faqs
Specific Gravity Definition Calculation Solved Examples Faqs
Specific Gravity of gases is normally calculated with reference to air - and defined as the ratio of the density of the gas to the density of the air - at a specified temperature and pressure.

Specific gravity to density calculator. The specific gravity is a unitless number that compares the density of a substance to the density of some standard substance usually but not always water. ρ 0 Density of pure water 1000 kgm-3 4C SG Specific gravity eg. The calculator below can be used to estimate the density and specific weight of ethylene at given temperature and pressure.
Order inline now Get free shipping. The conversion formula used by this tool is. See also Thermophysics properties of.
Specific gravity also referred to as relative density is the ratio of the density of a material compared to the density of water at 4 C 392 F. There is a wide range of instruments designed to measure the specific gravity. Specific Gravity calculator uses specific_gravity1 DensityWater Density to calculate the specific gravity of liquid The Specific Gravity formula is defined as the ratio between the density of an object and a reference substance.
We often see specific gravity as the ratio of the density of a material compared to the density of water. Density and Specific gravity Calculation. Specific gravity material density water density.
Online Ethylene Density Calculator. The specific gravity equation is. Calculating Brix from SG is based on an expression from a polynomial fit to a large data set.
Specific gravity is a ratio of density of a material to the density of. Because specific gravity is a ratio it is a unitless quantity. Density of substance when specific gravity is given calculator uses density specific gravity of the material1000 to calculate the Density The Density of substance when specific gravity is given formula is defined as the relative weight of that liquid compared to an equal volume of water.
Specific gravity is a ratio of the mass of a material to the mass of an equal volume of water at 4 o C. And we offer free techinical support for you. Brix 143254 sg 3 - 648670 sg 2 1125805 sg - 620389 Potential alcohol is calculated as.
Specific weight is given as Nm 3 and lb f ft 3. Causey explains density and specific gravity the relationship between them and how to calculate density and specific gravityhttpwwwyourCHEMcoachco. So to calculate the density of an object you just multiply its specific gravity by the density of water or the density of whatever the standard is.
SG ρ ρW. For example the specific gravity of water at 4 o C is 10 while its density is 10 gcm-3. The specific gravity can tell us based on its value if the object will sink or float in our reference substance.
Where SG specific gravity ρ density of the material kgm 3 ρW density of water kgm 3. The reference density of water at 4 o C 39 o F is used as the reference as these are the conditions of maximum density. Density is the mass of material per unit volume.
The Specific Gravity can be calculated as. Specific gravity is the density of a material divided by the density of water at 4C Therefore density of water 1000 kgm3 Specific gravity Density of. People usually choose that temperature as it is when water is at its densest.
SG ρ gas ρ air 3 where SG specific gravity of gas ρ gas density of gas kgm 3. The output density is given as kgm 3 lbft 3 lbgal US liq and slft 3. If another value such as Baume is provided it is first converted to specific gravity and then all other values are calculated from that.
Density o Eg. The density will be displayed as a specific gravity ratio in the lower text box. The formula for specific gravity.
Pure water 1 Density of Substance ρ This is the measure of the amount of mass per unit volume of a substance. The following formula is used to calculate the specific gravity of a material. Every value is calculated from specific gravity.
The specific gravity can be calculated as SG rgas rair rair 3 whereSG specific gas gravity gas density kgm3rair air density usually in NTP - 1204 kgm3 Molecular weights can be used to calculate specific gravity if gas and air densities are assessed at the same temperature and pressure. ρ Density of substance in kgm-3. SG ρ ρ 0.
Specific Gravity Calculator Enter the density of your material or substance to calculate its specific gravity. Density d is mass per unit volume of a substanceo It is usually expressed as gmL o The density of water may be expressed as 1 gmL o Density may be calculated by dividing mass by volume that is. Determine Specific Gravity given Density of Gasoline is 12 lbcu ft using the Specific Gravity Calculator in no time along with step by step procedure.
Density and is denoted by ρ symbol. Thus if 10 mL of sulfuric acid weighs 18 g its density is. Sino-Inst is manufacturer for Pressure Flow Level and temperature measurement control instruments.
Specific gravity is a ratio of densities where the density of an item is compared to divided by the density of a common substance.
These concepts are very useful in the food industry rubber industry and whole of the material science. Specific Gravity of gases is normally calculated with reference to air - and defined as the ratio of the density of the gas to the density of the air - at a specified temperature and pressure.
 Density And Specific Gravity Youtube
Density And Specific Gravity Youtube
Define density specific weight and specific gravity.

Relationship between specific gravity and density. Specific gravity is commonly divided a reference material which is usually water. Density is the material per unit volume while specific gravity is the ratio of the density of a substance to the density of a reference substance. In this article we are going to discuss relative density and specific gravity in depth and about their applications definitions similarities and.
It is noteworthy that specific gravity is a. One gallon of mercury has a mass of 512 kg. So now Density Mass Volume.
Just like density temperature and pressure have an influence on specific gravity. You will also find this Nomograph useful. The Specific Gravity can be calculated as.
And this is why specific gravity does not have a standard unit. Weight of water. In the English system of units the density of water is about 624 lbft 3 so the near equality between specific gravity and density is.
S specific gravity of slurry Si specific gravity of liquid phase Ss specific gravity of solids phase Cw concentration of solids by weight Cv concentration of solids by volume EXAMPLE. Volume of soil solid in a given soil mass. Weight of soil solid in a given soil mass.
The density units cancel leaving specific gravity a unitless number. In the industries specific gravity is used to measure concentrations of solutions. In this video we will discuss about the difference between specific gravity and density.
Specific gravity is the density of a substance divided by the density of water. 1 MINERAL PROCESSING FORMULAS. Since water has a density of 1 gramcm³ at sea level and 4C specific gravity is usually very close to the same value as density but without any units.
Density is defined as the factor which shows the measurement. The key difference between density and specific gravity is that density is an absolute value while specific gravity is a relative value for a substance. Density provides an absolute measure whereas specific gravity.
Whereas density is represented in the units for weight relative to the size. When compared to density specific gravity does not have an SI unit. The main difference between density and specific gravity is that density is the mass per unit volume of the substance whereas specific gravity is a ratio comparing the density of one substance to the density of another reference substance.
A plate at 05 mm distance from another fixed plate moves at 025 ms and requires a force of 2 Pa to maintain this speed. 6 Zeilen There is a noticeable difference between density and specific gravity even though both are used. Write the relation between density and specific weight.
In this article we will make a formula or equation or relation between void ratio e water content w degree of saturation and specific gravity G. Difference between density and specific gravity Density and Specific gravity. Since we often assume the density of pure water to be 10 gmL the specific gravity usually agrees closely with density.
This means that density will only measure the density of an object depending on its mass while specific gravity is the density of the substance divided by the density of water. Both these concepts hold almost the same idea. If the liquid has a specific gravity of 12 and the concentration of solids by weight is 35 with the solids having a specific gravity of 22 then.
Recommended for youhello friends is video main density or specific gravity k. Specific gravity is using the expression of density in relation to the density of some standard or reference. While density is expressed as an absolute term specific gravity is considered a relative term.
Relative density and specific gravity are two concepts used in comparing densities of solids liquids and gas. 18 Zeilen Density vs. Because the density of water is very nearly 1 gcm 3 the density of any substance in gcm 3 is nearly the same numerically as its specific gravity relative to water.
Specific gravity is the density of a substance divided by the density of water. Usually water is a standard reference. The main difference between density and specific gravity is its use in measuring this mass of a liquid.
SG ρ gas ρ air 3 where SG specific gravity of gas ρ gas density of gas.