PH -log 10 a H. The acidity or basicity level defines the pH and pOH scale chart value of the neutral acidic or alkaline solution in chemistry.
 Ph Chemistry Acids Bases Definition Calculating Ph Value Videos Examples
Ph Chemistry Acids Bases Definition Calculating Ph Value Videos Examples
In chemistry pH denoting potential of hydrogen or power of hydrogen is a scale used to specify the acidity or basicity of an aqueous solution.

Definition of ph scale in chemistry. Similarly a pH of 11 is ten times more basic than a pH of 10. A pH of 7 is neutral. Aqueous solutions at 25C with a pH less than 7 are acidic while those with a pH greater than 7 are basic or alkaline.
The pH scale was developed by the Danish Sørensen in 1909. Chemical Geology 1981 32 1-4 207-219. The pH scale ranges from 0 to 14.
The pH scale is a way to determine the acidity or alkalinity of a substance. A pH greater than 7 is basic. PH is a measure of the hydrogen ion activity typically in aqueous solution.
Its a scale that determines whether a solution is acidic neutral or basic. Likewise a pH of 3 is one hundred times more acidic than a pH of 5. For example a pH of 3 is ten times more acidic than a pH of 4.
The pH scale usually ranges from 0 to 14. PH Scale The pH scale is used to determine the acidity or alkalinity of a substance. It represents the concentration of ions H in a solutionThe more ions a substance contains H the more acidic it is.
In pH the p stands for -log 10 and the H stands for hydrogen ion activity. PH scale range or concentration of H ion differentiate neutral acidic and basic solutions. Pure and Applied Chemistry.
PH-electrode measurements of single-ion activity coefficients consistent with a pH convention. Only a small amount of indicator compound is needed to produce a visible color change. Pure and Applied Chemistry Volume 57 Issue 3 1985 Source Title.
PH is a measure of hydrogen ion concentration a measure of the acidity or alkalinity of a solution. Well go through the definition of pH scale along with. Definition of pH scales standard reference values measurement of pH and related terminology Recommendations 1984 in.
What is pH scale in chemistry. The more it contains ions OH the more it is basic. PH quantitative measure of the acidity or basicity of aqueous or other liquid solutions.
Learn about the pH scale and take a look at some familiar foods and. Learn more about pH. The pH scale is used to determine the degree of acidity of a substance.
The pH-scale is normally between 0 and 14. The chemical properties of many solutions enable them to be divided into three categories - acidic alkaline and neutral solutions. Scientists use the pH scale to measure how basic alkaline or acidic things are.
The pH is a measure of the concentration of hydrogen ions the acidity or alkalinity of a solution. The pH scale is logarithmic meaning that an increase or decrease of an integer value changes the concentration by a tenfold. PH scale - from potential of Hydrogen the logarithm of the reciprocal of hydrogen-ion concentration in gram atoms per liter.
The term widely used in chemistry biology and agronomy translates the values of the concentration of the hydrogen ion into numbers between 0 and 14. The pH scale measures how acidic or basic a substance is. Values range from 0 to 14 where pH equals 7 is neutral below 7 is acid and above 7 is basic.
Provides a measure on a scale from 0 to 14 of the acidity or alkalinity of a solution where 7 is neutral and greater than 7. PH scale range. A pH indicator or acid-base indicator is a compound that changes color in solution over a narrow range of pH values.
What is the pH scale. When used as a dilute solution a pH indicator does not have a significant impact on the acidity or alkalinity of a chemical solution. Acidic solutions solutions with higher concentrations of H ions are measured to have lower pH values than basic or alkaline solutions.
 Definition Of Ph Scale In Chemistry Chemswot Com
Definition Of Ph Scale In Chemistry Chemswot Com
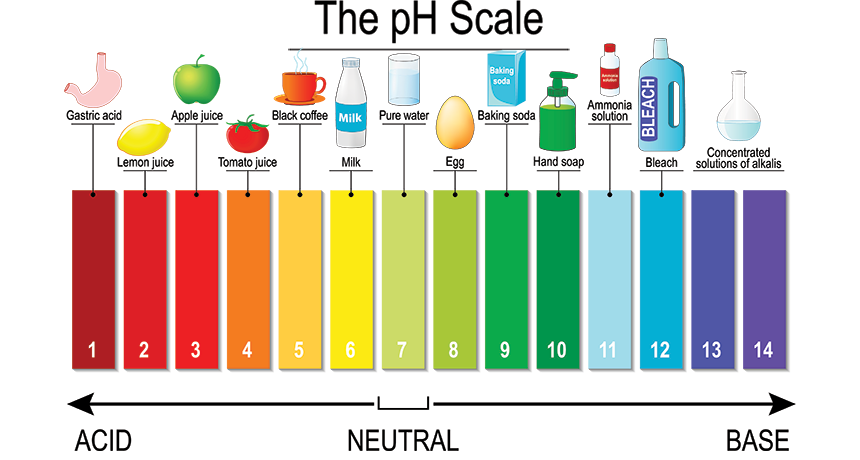 Scientists Say Ph Science News For Students
Scientists Say Ph Science News For Students
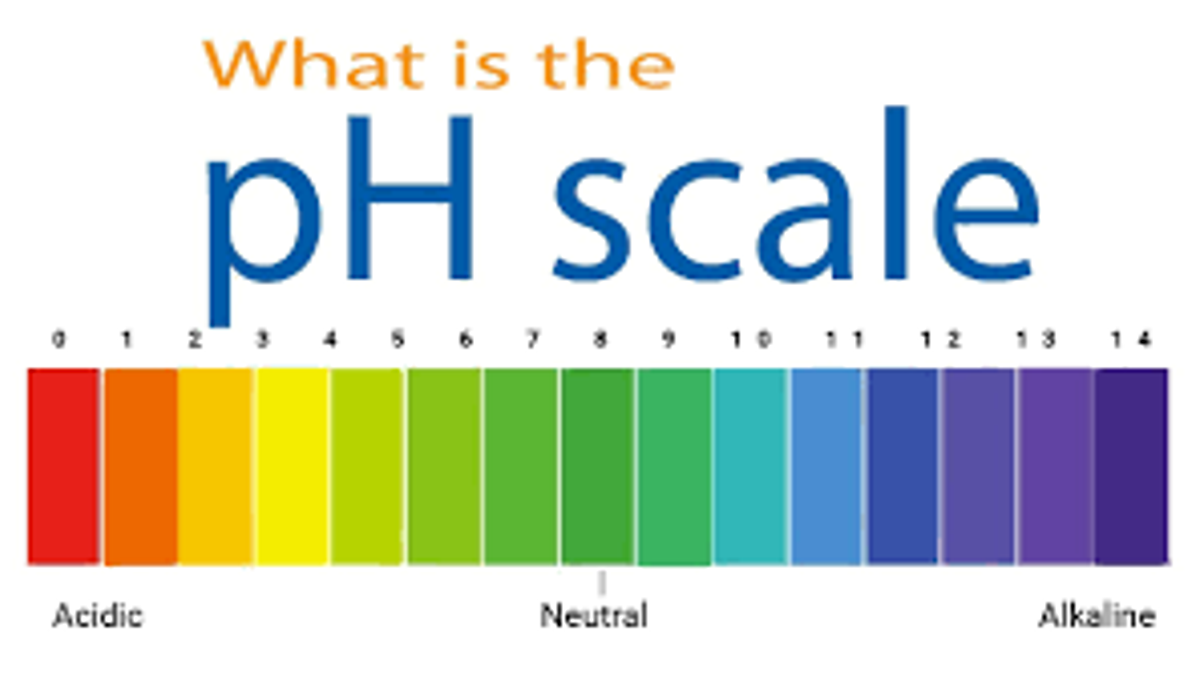 The Concept And Importance Of Ph Scale
The Concept And Importance Of Ph Scale
Bsl Chemistry Glossary Ph Scale Definition
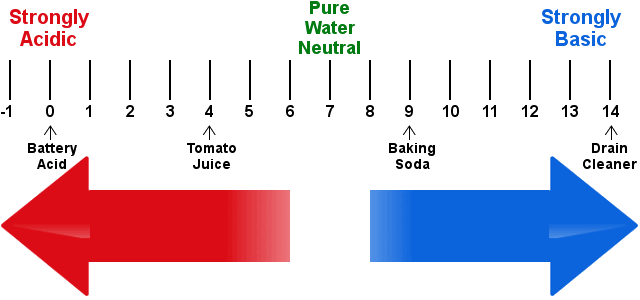 Definition Of Ph Chemistry Dictionary
Definition Of Ph Chemistry Dictionary

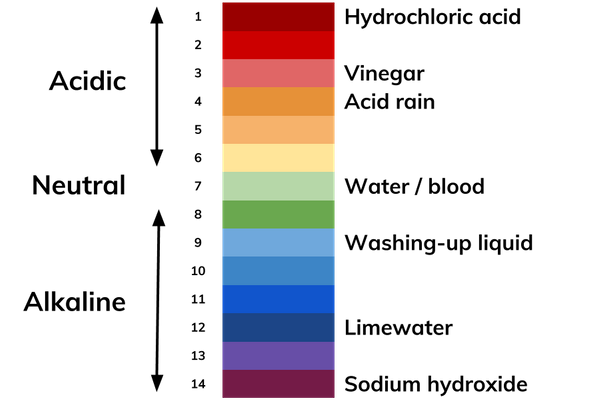
/definition-of-ph-in-chemistry-604605_final-5c8fac8446e0fb00017700d1.png)