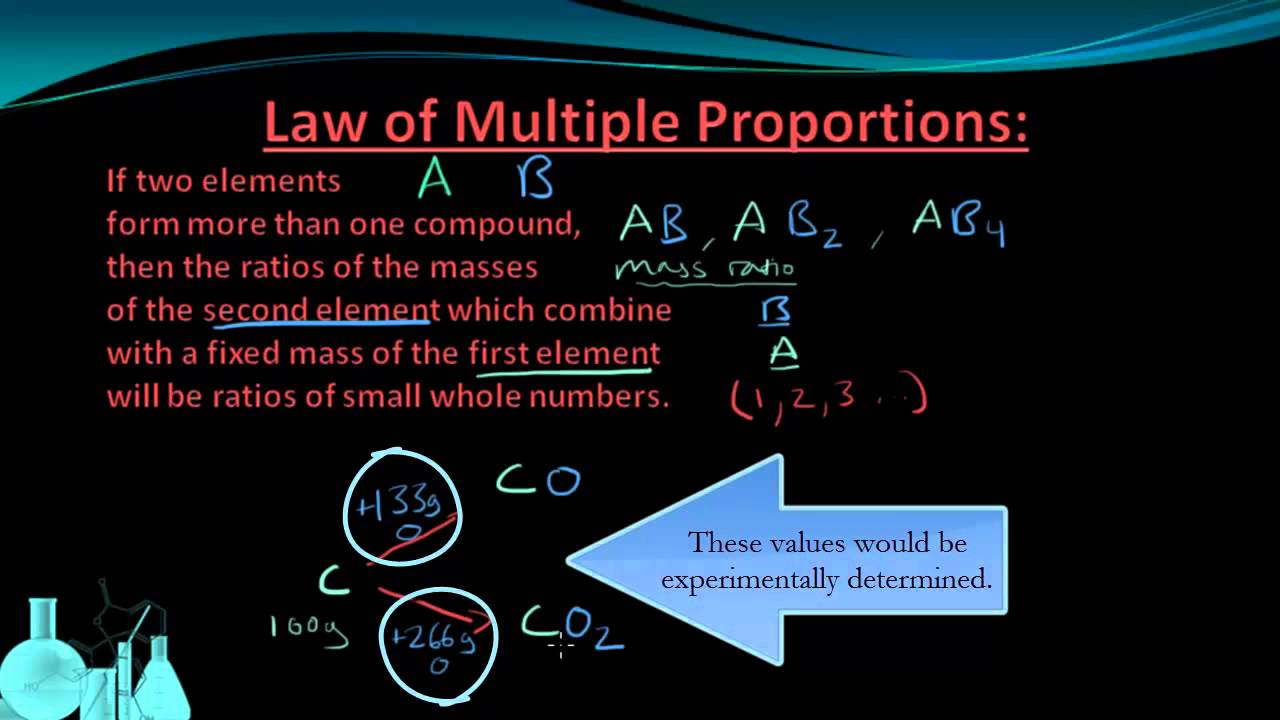
Once the idea that elements combined in definite proportions to form compounds was. For each of the two you can as you realised set up a law of definite proportions which often gives you a rather weird rational ratio.
 2 5 The Law Of Multiple Proportions And Dalton S Atomic Theory Chemistry Libretexts
2 5 The Law Of Multiple Proportions And Dalton S Atomic Theory Chemistry Libretexts
Numerical on multiple proportions - definition For carbon dioxide 266g of oxygen100g of carbon 266.

Law of multiple proportions. According to the law of multiple proportions if two elements chemically combine with each other forming two or more compounds with different compositions by mass then the ratios of masses of two interacting elements in the two compounds are small whole numbers. A H_2S and SO_2 are not example of law of multiple proportion because elements are different. Law of multiple proportions statement that when two chemical elements combine with each other to form more than one compound the weights of one element that combine with a fixed weight of the other are in a ratio of small whole numbers.
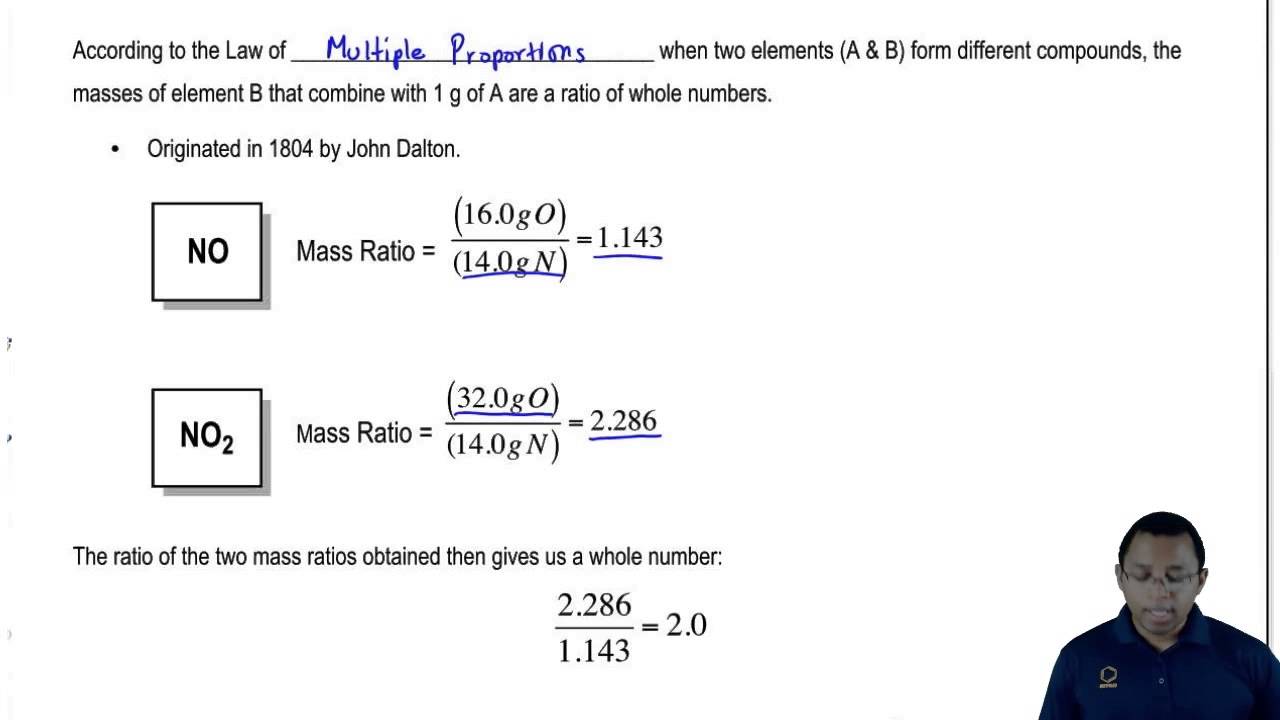
For example Dalton knew that the carbon element forms two oxides by. The law of multiple proportions states that when two elements combine to form more than one compound the mass of one element which combines with a. If your ratio came out like 2109 then youd know to round to the nearest whole number and work from there.
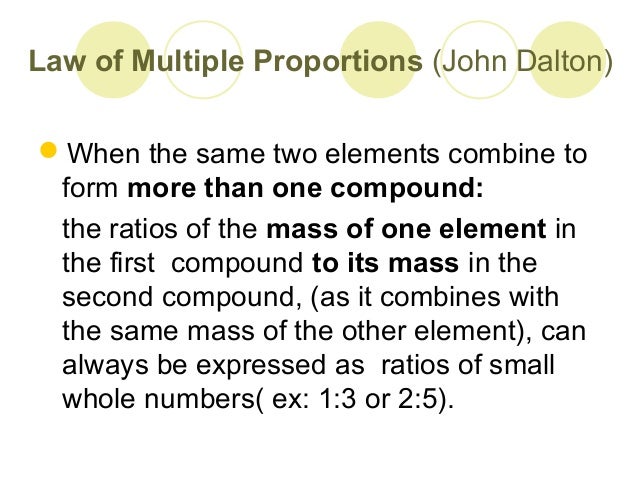
John Dalton formulated the law of multiple proportions as part of his theory that atoms formed the basic indivisible. 3 рядків The law of multiple proportions was given by British scientist John Dalton in 1803. Law of multiple proportions - law When two elements combine with each other to form two or more compounds the ratios of the masses of one element that combines with the fixed ratio of the other are simple whole numbers.
For example iron can react with chlorine to give two different chlorides. Consider the example S. Solving Law of Multiple Proportions Problems.
The law can be illustrated by the composition of the five oxides of nitrogen. State the Law of Multiple Proportion. About Law of Multiple Proportions and Example.
While the ratio in this example problem worked out to be exactly 21 its more likely chemistry problems and real data will give you ratios that are close but not whole numbers. The law of multiple proportions says that when elements form compounds the proportions of the elements in. Law of Multiple Proportions Law of Multiple Proportions.
The law of multiple proportions now states that some elements are special. John Dalton FRS 6 September 1766 27. Whenever the same two elements form more than one compound the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.
According to law of multiple proportions when two elements combine to form more than one compound the fixed amount of one element combining with other element have simple whole number ratio. Law of multiple proportions. The law of multiple proportions states that whenever the same two elements form more than one compound the.
Democritus has first suggested the atoms existence. This law states that when two elements combine together to form more than one compound the weights of one element that unite with a given weight of the other are in the ratio of small whole numbers. Learn more about the law of multiple proportions in this article.
The law of multiple proportions states that when two chemical elements combine with each other to form more than one compound the weights of one element that combine with a fixed weight of the other are in a ratio of small whole numbers. According to Wikipedia John Dalton. Key Points The law of multiple proportions is a rule of stoichiometry.
Law of Multiple Proportions was given by John Dalton in 1804. Who identified the.

 Law Of Multiple Proportions Youtube
Law Of Multiple Proportions Youtube
 Law Of Multiple Proportions Chemistrygod
Law Of Multiple Proportions Chemistrygod
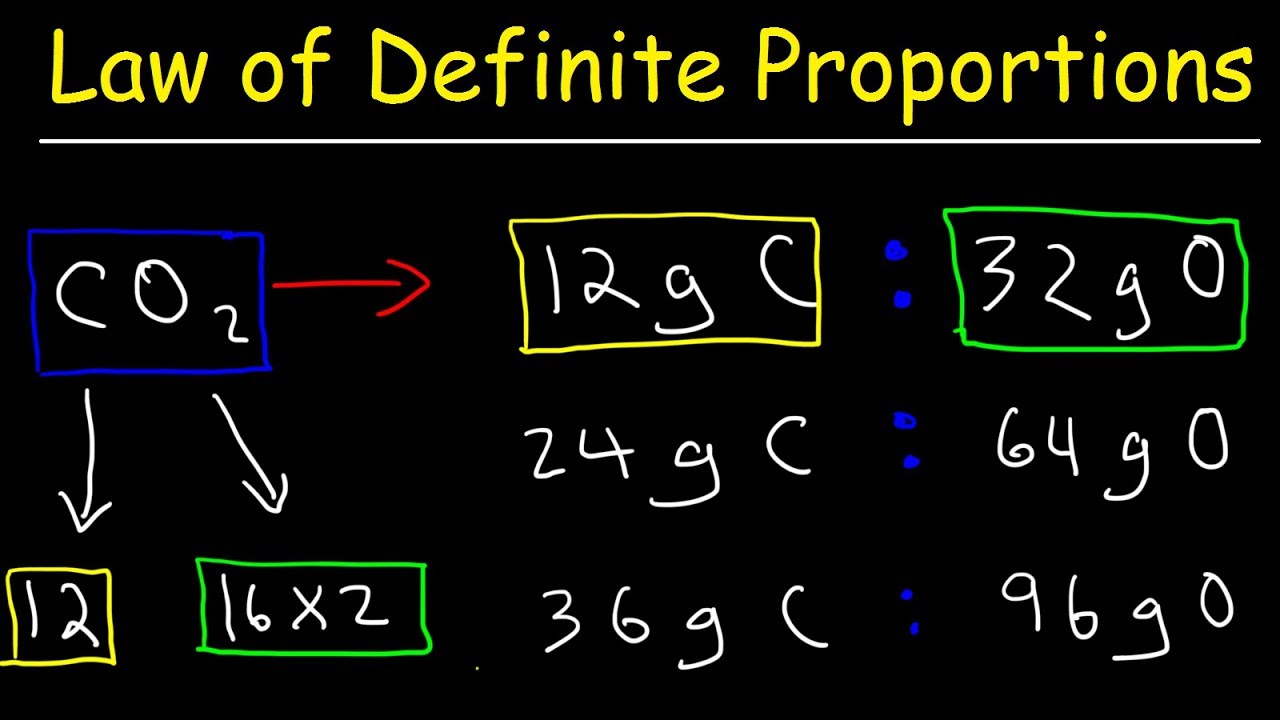 Law Of Definite Proportions Chemistry Practice Problems Chemical Fundamental Laws Youtube
Law Of Definite Proportions Chemistry Practice Problems Chemical Fundamental Laws Youtube
 Law Of Multiple Proportions And Law Of Definite Proportions
Law Of Multiple Proportions And Law Of Definite Proportions
 Law Of Multiple Proportions Definition Examples Science Class 2021 Video Study Com
Law Of Multiple Proportions Definition Examples Science Class 2021 Video Study Com
 Law Of Multiple Proportions In Chemical Combination John Dalton In Hindi Youtube
Law Of Multiple Proportions In Chemical Combination John Dalton In Hindi Youtube