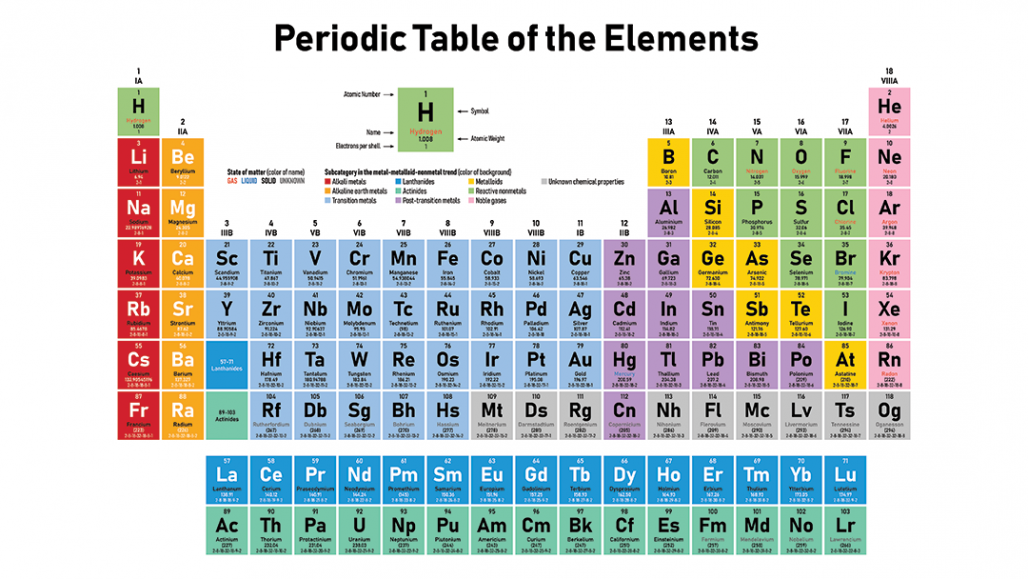
The arrangement of elements in the Periodic table starts from the very first top left corner. Columns are called groups.
 How The Elements Are Laid Out In The Periodic Table Chemistry The Fuse School Youtube
How The Elements Are Laid Out In The Periodic Table Chemistry The Fuse School Youtube
How are the elements arranged on the Periodic Table.

How are elements arranged on the periodic table. He was also smart enough to leave space for new elements that he thought would be discovered and he was right. A table in which the chemical elements are arranged in order of increasing atomic number. Going down the periodic table the number of atomic orbitals increases by one for each row.
British scientist Henry Mosely. In the modern periodic table elements are arranged by atomic number. The columns of the table are called groups some of which have specific names such as the noble gases and the halogens.
The periodic table tells scientists how reactive an element is if it is a metal nonmetal or metalliod and what group its in. The first element with atomic number 1 ie hydrogen is placed in the first cell then gradually the elements with atomic number 2 3 4 upto 118 are placed from the left to right in. He also predicted the properties of five unknown elements and what their compounds would be even before their discovery based on the principles of the periodic table he had arranged.
Mendeleev arranged the elements both in terms of their atomic weights and valence. Elements are arranged from left to right and top to bottom in order of increasing atomic number. Atomic numbers were first.
The atomic number is the number of protons and electrons in an electrically neutral atom of an element. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesthe structure of the table shows periodic trendsthe seven rows of the table called periods generally have metals on the left and. Each element has its own unique atomic number which is the number of protons in the nuclei of its atoms and which defines the element.
The table is arranged in vertical columns called Groups numbered 1 8 and in rows called Periods numbered 1 7. The elements in the Modern Periodic table are arranged in the increasing order of their Atomic Number. This element is hydrogen having atomic number 1.
Elements with similar properties are arranged in the same column called a group and elements with the same number of electron shells are arranged in the same row called a period. All periods begin with an alkali metal in group 1 and end with a noble gas in group 188A. They are also arranged in vertical columns known as groups with these groupings based on shared properties.
Elements in the group 1A have one electron in the last shell. Atoms with the same atomic number make up a chemical element. Let me show you how they are arranged As shown in the above image the element with a minimum atomic number is placed in the top-left corner of the Periodic table.
The Periodic table elements are arranged in the increasing order of their atomic number. Order generally coincides with increasing. The elements are arranged by energy levels valence electrons what group its on Alkali Metals Alakine Earth Metals Boron Family etc and forms metals nonmetals metalliods.
How are elements primarily arranged on the periodic table. They are placed with the lowest atomic number first and elements with increasing atomic numbers run to the right. The vertical columns are called groups.
In the periodic table elements are organized by atomic number. The elements are arranged by energy levels valence electrons what group its on Alkali Metals Alakine Earth Metals Boron Family etc and forms metals nonmetals metalliods. The periodic table got its name from the way the elements are arranged in rows which are called periods.
Order generally coincides with increasing atomic mass. The periodic table of elements arranges all of the known chemical elements in an informative array. The horizontal rows are called periods.
Elements are arranged on the Periodic Table in order of increasing atomic number where each element has one proton more than the element preceding it. If playback doesnt begin shortly try restarting your device. The elements are arranged in order of increasing atomic number.
The elements are arranged into 18 groups columns and seven periods rows by increasing atomic number. In the periodic table the elements are arranged in columns and rows according to increasing atomic number atomic number often represented by the symbol Z the number of protons in the nucleus of an atom as well as the number of electrons in the neutral atom. Elements are arranged from left to right and top to bottom in order of increasing atomic number.
The periodic table of elements arranges all of the known chemical elements in an informative array. Periodic table and element. The periodic table tells scientists how reactive an element is if it is a metal nonmetal or metalliod and what group its in.
Videos you watch may be added to the TVs watch history and. The Correct Answer is they are primarily arranged by atomic number of protons.
 Periodic Table Of Elements Live Science
Periodic Table Of Elements Live Science
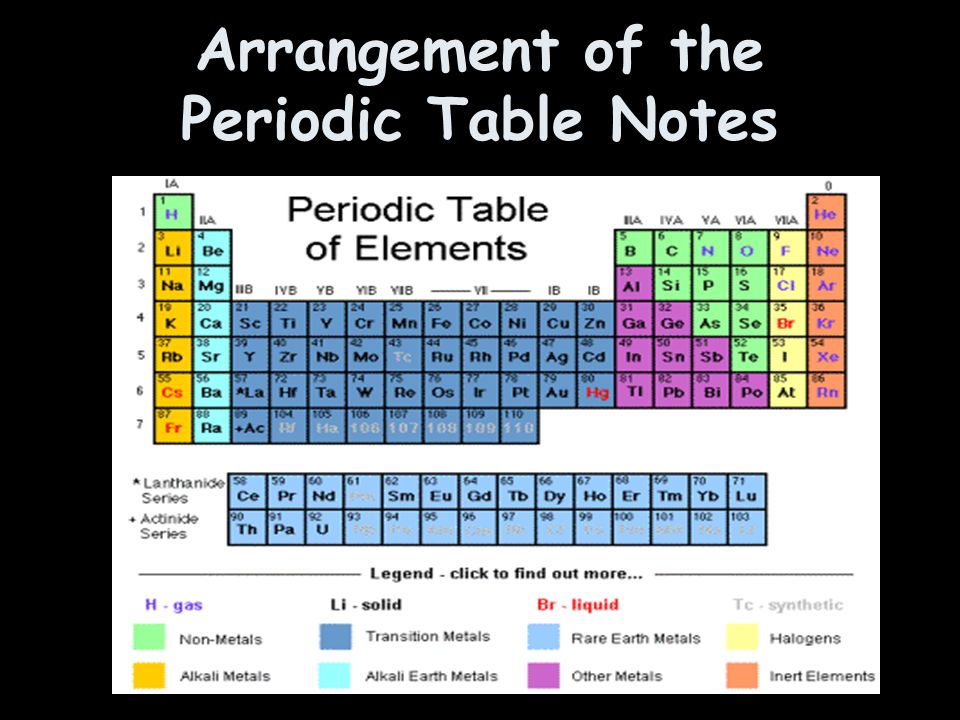 Arrangement Of The Periodic Table Notes Elements Are Made Up Of All The Same Atoms Identified By It S Atomic Of Protons Arrangement Of Valence Ppt Download
Arrangement Of The Periodic Table Notes Elements Are Made Up Of All The Same Atoms Identified By It S Atomic Of Protons Arrangement Of Valence Ppt Download
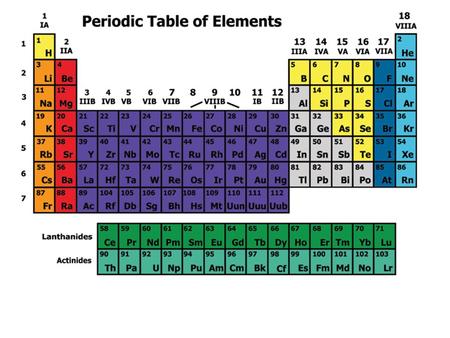 The Modern Periodic Table Elements Arranged In Order Of Increasing Atomic Number Arranged In Groups And Periods Ppt Download
The Modern Periodic Table Elements Arranged In Order Of Increasing Atomic Number Arranged In Groups And Periods Ppt Download
 Study The Image Above How Are The Elements Arranged In The Modern Periodic Table
Study The Image Above How Are The Elements Arranged In The Modern Periodic Table
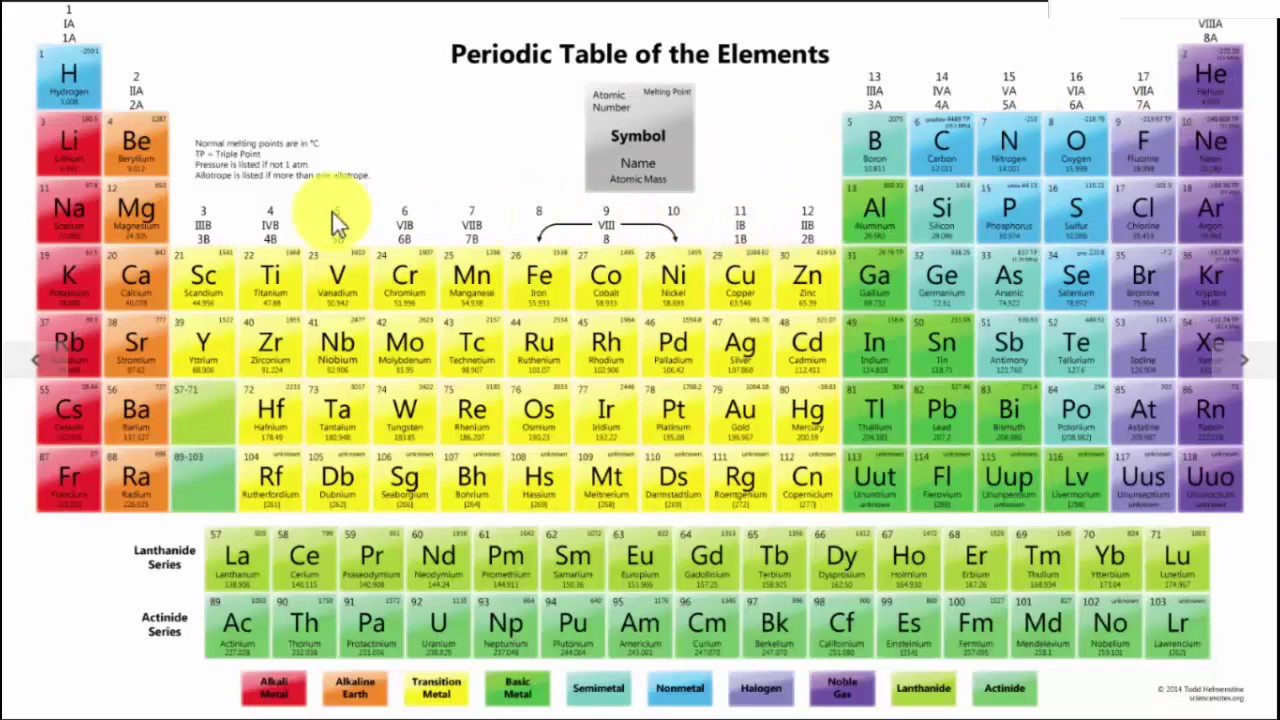 How Are The Elements In The Periodic Table Arranged
How Are The Elements In The Periodic Table Arranged
 411a M2 U2 P1 The Periodic Table
411a M2 U2 P1 The Periodic Table
 The Arrangement Of The Elements The Periodic Table Siyavula
The Arrangement Of The Elements The Periodic Table Siyavula
What Is The Arrangement Of The Periodic Table Based On Quora