Starch and cellulose are two common carbohydrates. Other organic molecules with similar formulas were found to have a similar ratio of hydrogen to oxygen.
 Iii Carbohydrates Structures And Types A Guide To The Principles Of Animal Nutrition
Iii Carbohydrates Structures And Types A Guide To The Principles Of Animal Nutrition
This composition gives carbohydrates their name.
Chemical formula for carbohydrates. Carbohydrates consist of the elements carbon hydrogen and oxygen with a. In the early part of the 19th century substances such as wood starch and linen were found to be composed mainly of molecules containing atoms of carbon C hydrogen H and oxygen O and to have the general formula C 6 H 12 O 6. The double-sugar units are known as disaccharides.
They are made up of carbon carbo - plus water - hydrate. Glycosides formed from glucose are called glucosides. Carbohydrates are basically compounds made up of carbon oxygen and Hydrogen.
The general formula for carbohydrate is C x H 2 O y. Carbohydrates Carbohydrates have the general molecular formula CH 2 O and thus were once thought to represent hydrated carbon. Carbohydrate general formula is CnH2nOn eg.
We have now determined symbols and formulas for many ingredients in chemical equations involving water and carbohydrates but one important step remains. The scientific term for a single sugar is monosaccharide. Although it must be remembered that this is just a general formula.
However the arrangement of. For example glucose is C 6 H 12 O 6 and maltose is C 12 H 22 O 11. Carbohydrates have the general molecular formula CH2O and thus were once thought to represent hydrated carbon.
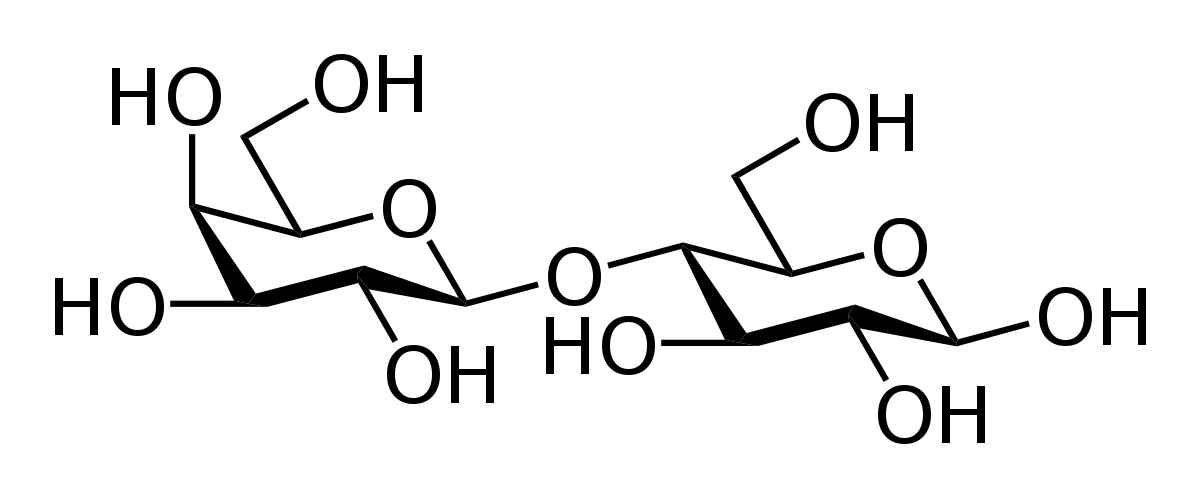
Many sugar molecules can join together in. In chemistry carbohydrates are a common class of simple organic compounds. The general formula if carbohydrates is C x H 2 0 Y.
A polysaccharide may contain anywhere from a few monosaccharides to several thousand monosaccharides. Maltose is C 12 H 22 O 11. We must be sure that our equations reflect the way they occur in our bodies or our environment where all the atoms in the reactants also appear in products.
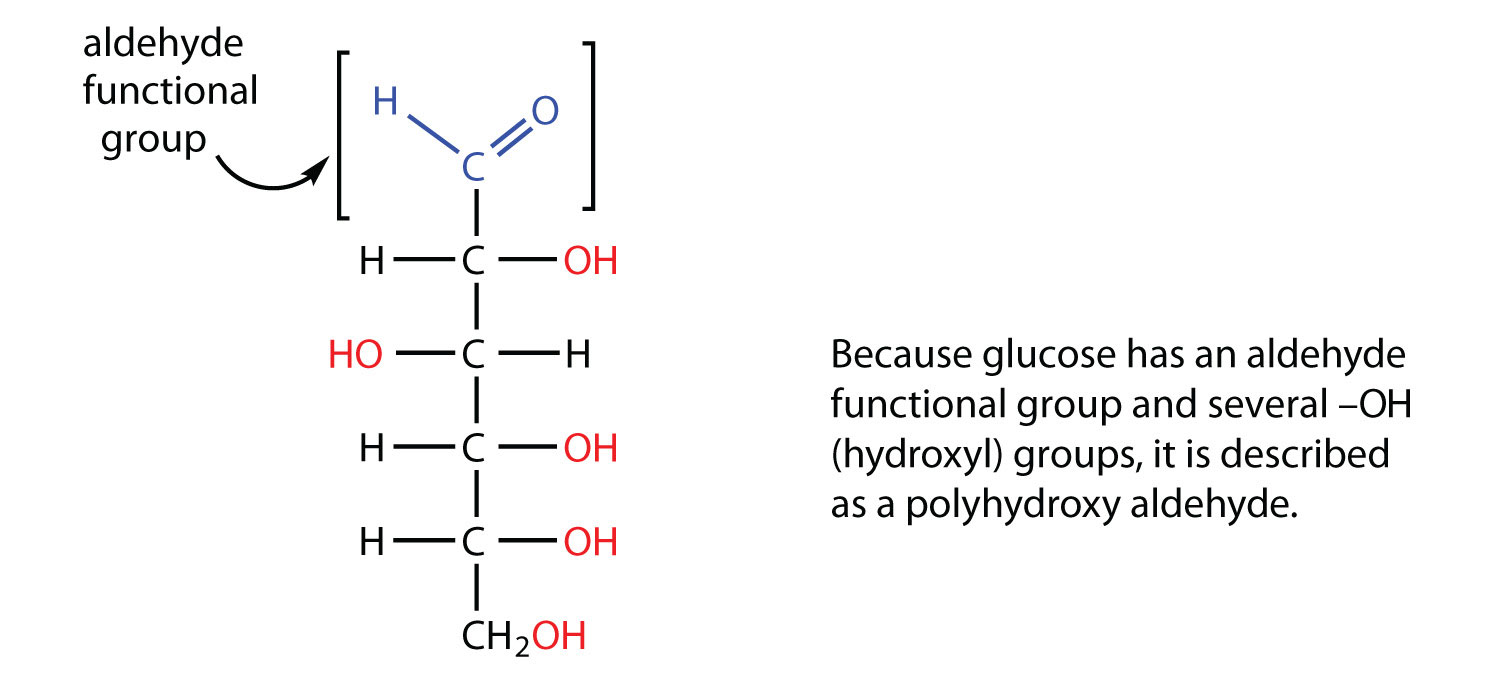
I need to know the CHEMICAL Not WORD equation for the breakdown of Carbohydrates Proteins Lipids by enzymes the actual products of the nutrients dig. The chemical formula for carbohydrate is carbon oxygen and hydrogen the number of atoms varies on what carbohydrate. A carbohydrate is an aldehyde or a ketone that has additional hydroxyl groups.
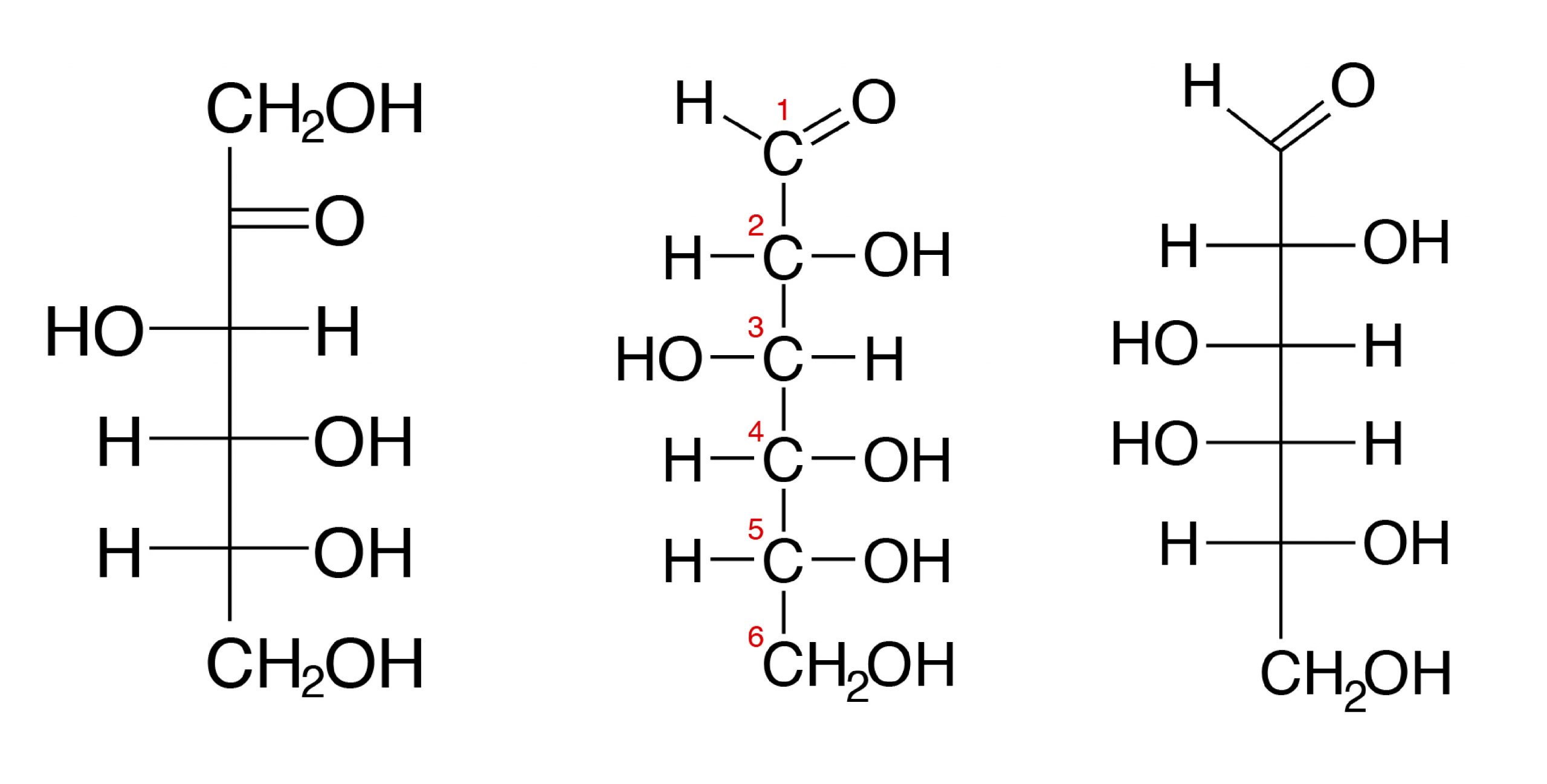
Glucose is C 6 H 12 O 6. Carbohydrates have the empirical formula CH 2 O n Fig. However the arrangement of atoms in carbohydrates has little to do with water molecules.
The term generated from carbon and hydrate. Glucose is the simplest monosaccharide and probably the most familiar sugar especially if you have been in the hospital. The simplest carbohydrates are called monosaccharides which have the basic structure CH 2 O n where n is three or greater.
Starches are one of the more common polysaccharides. Yuhan Zhang Proud A-level Biology student. Polysaccharides have a general formula of ceC_xH_2O_y where x is usually a large number between 200 and 2500.
All carbohydrates have something in common. Carbohydrates are biological molecules made of carbon hydrogen and oxygen in a ratio of roughly one carbon atom to one water molecule. Many carbs have the general chemical formula CxH2Ox but the class is too large to fit into a simple chemical formula.
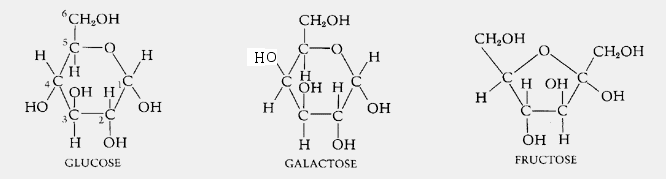
Carbohydrates is a large family of a variety of compounds so there is no single formula for carbohydrates. Both are macromolecules with molecular weights in the hundreds of thousands. The most common monosaccharides hexoses are glucose galactose and fructose.
Two monosaccharides link together to form a disaccharide. Polysaccharides are also called complex carbohydrates. The general formula for any carbohydrate is CH 2 O x where x is any number between three and eight.
There are various exceptions to this that we will see. Though some also contain n itrogen phosphorus or sulfur. They are built out of sugar molecules.
Sugar molecules can exist separately as single units or they can join together in pairs to form double sugars. The hydroxyl group that is attached to the anomeric carbon atom ie the carbon containing the aldehyde or keto group of carbohydrates in solution has unusual reactivity and derivatives called glycosides can be formed. The general formula if carbohydrates is C x H 2 O y.
Carbohydrates are often isomers -.
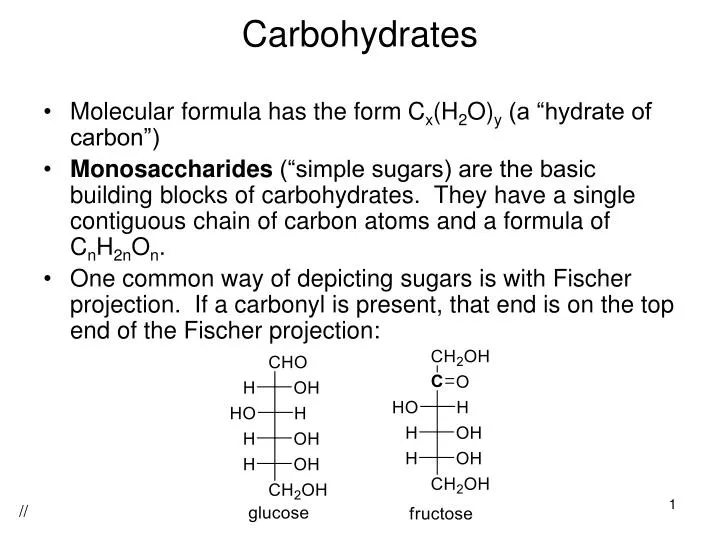 Ppt Carbohydrates Powerpoint Presentation Free Download Id 2169867
Ppt Carbohydrates Powerpoint Presentation Free Download Id 2169867
 Biomolecules Carbohydrates Lhs Cns Chemistry Term A
Biomolecules Carbohydrates Lhs Cns Chemistry Term A
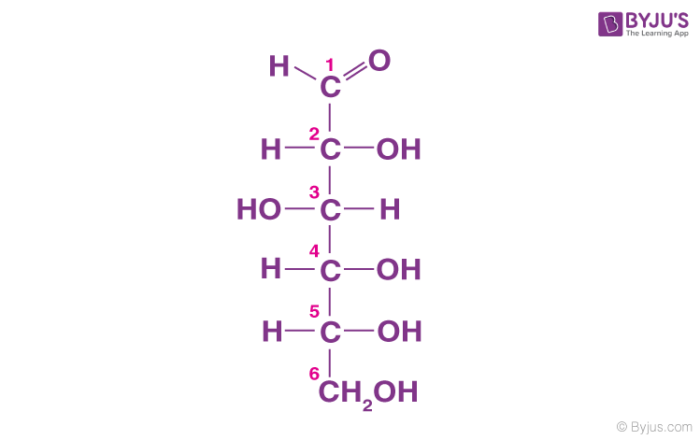 Classification Of Carbohydrates With Definition Types Structure Formula With Examples
Classification Of Carbohydrates With Definition Types Structure Formula With Examples