This law is applicable when two elements combine to give more than one chemical compound. The law of multiple proportions states that when two elements combine to form more than one compound the mass of one element which combines with a fixed mass of the other element will always be.
 Law Of Multiple Proportions Chemistry For Non Majors
Law Of Multiple Proportions Chemistry For Non Majors
The law of multiple proportions was formulated by John Dalton in 1804.
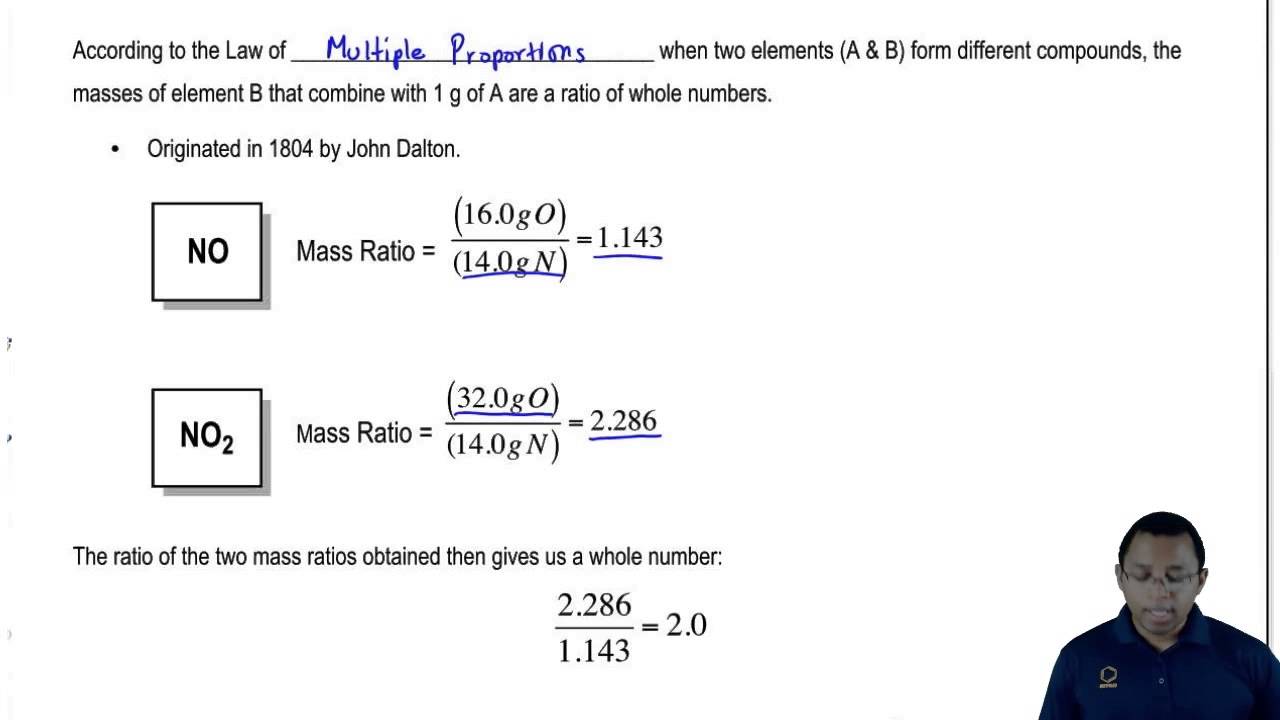
What is law of multiple proportions. AnswerIn chemistry the law of multiple proportions states that if two elements form more than one compound between them then the ratios of the masses of the. The law states that When two elements combine to form two or more compounds the masses of one element which combined with a fixed mass of the other bear a simple ratio to one another. It states that the masses of one element which combine with a fixed mass of the second element are in a ratio of whole numbers.
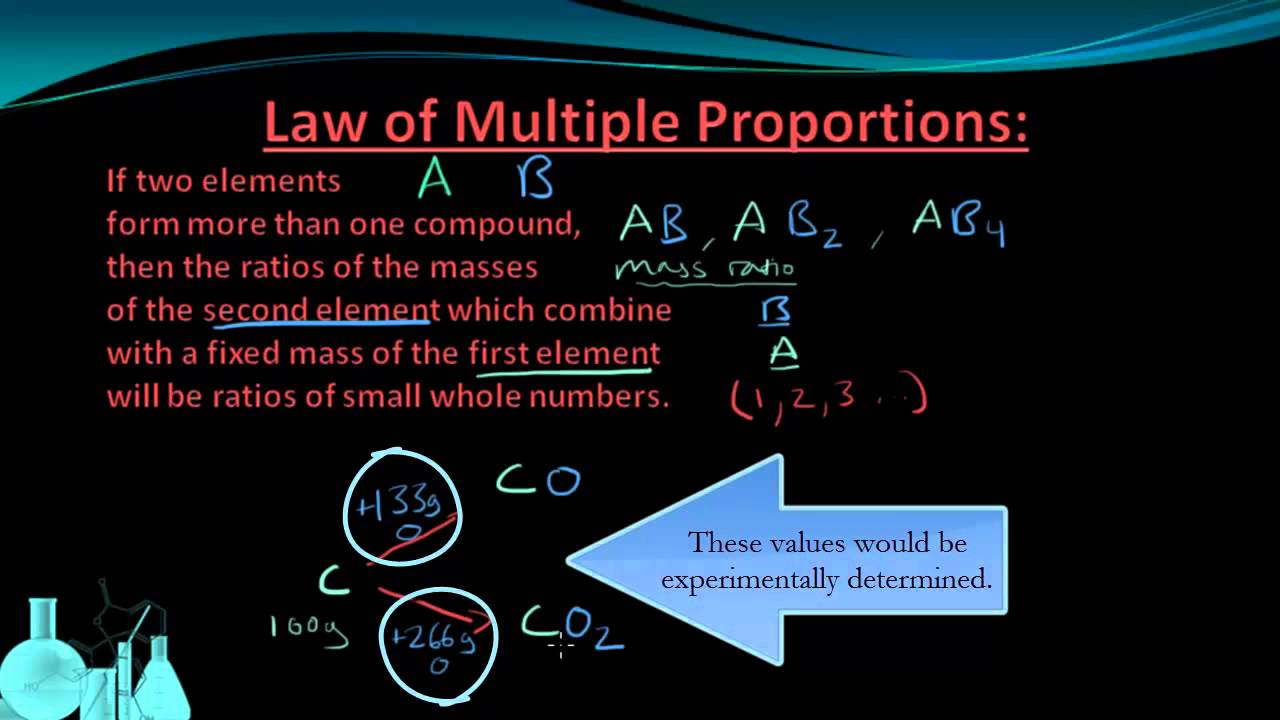
For carbon monoxide 133 g of oxygen100 g of carbon 133. Law of multiple proportions statement that when two chemical elements combine with each other to form more than one compound the weights of one element that combine with a fixed weight of the other are in a ratio of small whole numbers. Whenever the same two elements form more than one compound the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.
As I have shown above the Law of Multiple Proportions is a corollary of the Law of Definite Proportions. We can call this law Daltons law as well because the law was developed by John Dalton in 1803. For example in water H 2 O the ratio of.
The Law of Multiple Proportions The Law of Definite Proportions or Prousts Law states that in a single chemical compound such as H 2 O or CO the ratio of its component elements is a fixed whole number ratio. Law of Multiple Proportions Law of Multiple Proportions. Once the idea that elements combined in definite proportions to form compounds was.
The Law of Multiple Proportions deals with elements that form more than one compound. The two ratio are in the proportion 266133 21There for the ratio of masses of oxygen that combine with the same mass of carbon is 21 ie. It is regarded as a very important law in chemistry as it.
Law of multiple proportions. The Law of Multiple Proportionsstates that the masses of one element which combine with a fixed mass of the second element are in a ratio of whole numbers. An Explanation of the Law of Multiple Proportions This is sometimes called Daltons law.
For example carbon and oxygen react to form two compounds. This video explains what the Law of Multiple Proportions says about chemical formulas and uses an example with Carbon and Oxygen to demonstrate its meaning. John Dalton formulated the law of multiple proportions as part of his theory that atoms formed the basic indivisible.
The law of multiple proportions states that when two elements combine with each other to form more than one compound then the weights of one element that combine with a fixed weight of the other are in a ratio of small whole numbers. The law of multiple proportions was given by British scientist John Dalton in 1803. The law of multiple proportions states that whenever the same two elements form more than one compound the.
Key Points The law of multiple proportions is a rule of stoichiometry. X y c 3 Since a real constant can always be expressed as the ratio of two whole numbers this gave us the Law of Multiple Proportions. Law of multiple proportions states when two elements combine with each other to form more than one compound then the weights of one element that combine with a fixed weight of the other are in a ratio of small whole numbers.
This law was put forward by Dalton in 1803. Laptop162 laptop162 08102020 Biology Secondary School What is law of multiple proportion 2 See answers. Learn more about the law of multiple proportions in this article.
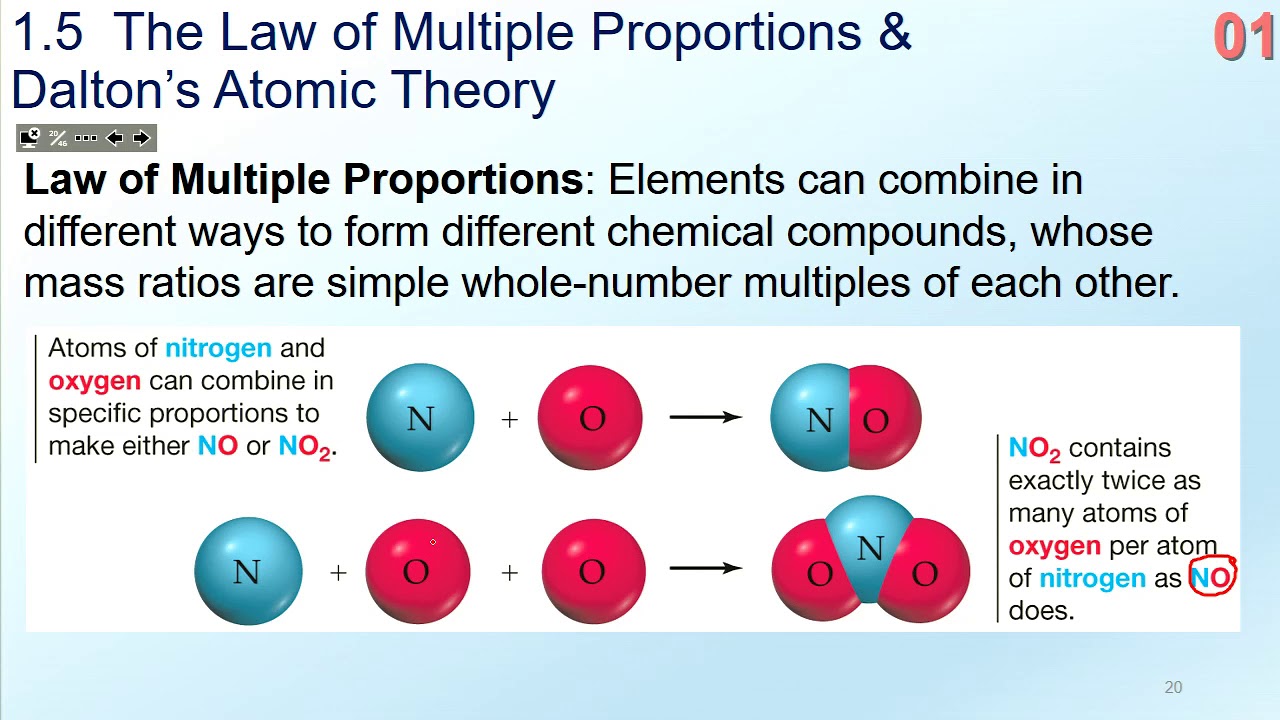
It states that the masses of one element that combine with a fixed mass of the second element are in a small whole number ratio. The law of multiple proportions says that when elements form compounds the proportions of the elements in. The law of multiple proportions is also observed in the formation of two oxides of nitrogen namely NO and NO2.
Therefore the masses of oxygen in the two compounds that combine with a fixed mass of carbon should be in a whole number ratio. When two elements combine to form more than one compound then the different weights of one element combining with a fixed weight of the other element are in. Hence the masses of oxygen in the two compounds that combine with a fixed mass of carbon should be in a whole-number ratio.
Numerical on multiple proportions - definition For carbon dioxide 266g of oxygen100g of carbon 266. The law of multiple proportions is the third postulate of Daltons atomic theory. In the first compound A 429 g of C react with 571 g of O.
Who identified the. Since the ratio of two real constants is another real constant we can express this as.
 Law Of Multiple Proportions Youtube
Law Of Multiple Proportions Youtube
 2 5 The Law Of Multiple Proportions And Dalton S Atomic Theory Chemistry Libretexts
2 5 The Law Of Multiple Proportions And Dalton S Atomic Theory Chemistry Libretexts
The Law Of Multiple Proportions Dalton S Law
 Law Of Multiple Proportions Chemistrygod
Law Of Multiple Proportions Chemistrygod
 Law Of Multiple Proportions Definition Examples Video Lesson Transcript Study Com
Law Of Multiple Proportions Definition Examples Video Lesson Transcript Study Com
 Chemistry 5 8b Law Of Multiple Proportions Youtube
Chemistry 5 8b Law Of Multiple Proportions Youtube
 Law Of Multiple Proportions Practice Problems Chemistry Examples Fundamental Chemical Laws Youtube
Law Of Multiple Proportions Practice Problems Chemistry Examples Fundamental Chemical Laws Youtube

 Ch 1 5 Law Of Multiple Proportions Daltons Atomic Theory Youtube
Ch 1 5 Law Of Multiple Proportions Daltons Atomic Theory Youtube